 15.09.2012, 10:22
15.09.2012, 10:22
|
Язык оригинала: Русский
#1
|
|
Гуру
Регистрация: 26.07.2008
Адрес: РФ, Самара
Сообщений: 75,397
Спасибо: 27,867
Поблагодарили 55,330 раз(а) в 24,283 сообщениях
Репутация: 102390
|
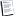 Find out why the "Flowers in Blue Vase" by Van Gogh darkened
Find out why the "Flowers in Blue Vase" by Van Gogh darkened
Цитата:
Asters in a painting by Vincent Van Gogh's "Flowers in Blue Vase" gradually turned from bright yellow to brown due to chemical reaction between the "lemon" pigment, cadmium sulfide, and a protective lacquer, according to the article published in the journal Analytical Chemistry.
Vincent Van Gogh painting "Flowers in a Blue Vase" in 1887, in the twilight of the Paris period of life of this great artist. In this painting depicted a bright yellow daisies and white anemones in a vase placed on the table. After the death of van Gogh, this picture was covered with varnish to enhance the conservation and bought in the early 20th century, Kröller-Müller Museum in the Netherlands Otterlo, RIA "Novosti".
Group of chemists led by Kuhn Janssens of the University of Antwerp (Belgium) has turned its attention to the picture after restorers discovered an unusual attack on some parts still life.
As the scientists explain, the yellow pigment - cadmium sulfide - gradually loses its brightness in contact with air. This is because the oxygen molecules oxidize sulfur and make the connection to sulfate, sulfate (SO4). Cadmium sulfate forms a white translucent film on the surface of the paint, making it fades and loses its usual brightness.
In the case of "Flowers in Blue Vase" raid was unusual - it was not the light, and dark, and its microstructure was not similar to cadmium sulfate crystals. Puzzled museum staff sent a small piece of paint from the painting to the lab and asked Janssens chemists determine the nature and cause of the formation of plaque.
Researchers enlightened paint samples using X-ray spectroscopy at the European synchrotron center in Grenoble and a German laboratory DESY and compared them to the "normal" bloom of cadmium sulfate.
"It turned out that the sulfate anions found the right partner for the reaction in the ions lead paint and therefore on the border between the paint and varnish formed gray and maloprozrachnye anglesite crystals - lead sulfate. His source, apparently, was the substance drier, which was added to coat with cover picture, "- said one of the participants in the work of German Gerald Falkenberg synchrotron center DESY.
According to scientists, the effect is compounded by the fact that cadmium ions devoid of sulfur react with other components of the paint and turned into cadmium oxalate (CdC2O4) - a combination of oxygen, cadmium and carbon. A combination of lead salts and cadmium oksolata and is the raid, which turned bright yellow daisies in a painting by Van Gogh in a mixture of orange and dark brown smears.
"Many of the paintings that Vincent wrote during the Paris period, were covered with lacquer and improper removal of this cover is one of the most difficult tasks faced by the restorers of Van Gogh in all countries of the world. Information provided by you Janssens and his colleagues, vital to making tough decisions that we have to do at times when such a complex cleaning of paintings "- added Restorer Ella Hendriks of the Van Gogh Museum in Amsterdam (Netherlands).
|
(a)
/////////
|

|

|

 Похожие темы
Похожие темы








 Древовидный вид
Древовидный вид